Polymers
1. Introduction
Prior to the early 1920's, chemists doubted the existence of molecules having molecular weights greater than a few thousand. This limiting view was challenged by Hermann Staudinger, a German chemist with experience in studying natural compounds such as rubber and cellulose. In contrast to the prevailing rationalization of these substances as aggregates of small molecules, Staudinger proposed they were made up of macromolecules composed of 10,000 or more atoms. He formulated a polymeric structure for rubber, based on a repeating isoprene unit (referred to as a monomer). For his contributions to chemistry, Staudinger received the 1953 Nobel Prize. The terms polymer and monomer were derived from the Greek roots poly (many), mono (one) and meros (part).
Recognition that polymeric macromolecules make up many important natural materials was followed by the creation of synthetic analogs having a variety of properties. Indeed, applications of these materials as fibers, flexible films, adhesives, resistant paints and tough but light solids have transformed modern society. Some important examples of these substances are discussed in the following sections.
Polymers are substances containing a large number of structural units joined by the same type of linkage. These substances often form into a chain-like structure. Polymers in the natural world have been around since the beginning of time. Starch, cellulose, and rubber all possess polymeric properties. Man-made polymers have been studied since 1832. Today, the polymer industry has grown to be larger than the aluminum, copper and steel industries combined.
2. Writing Formulas for Polymeric Macromolecules
The repeating structural unit of most simple polymers not only reflects the monomer(s) from which the polymers are constructed, but also provides a concise means for drawing structures to represent these macromolecules. For polyethylene, arguably the simplest polymer, this is demonstrated by the following equation. Here ethylene (ethene) is the monomer, and the corresponding linear polymer is called high-density polyethylene (HDPE). HDPE is composed of macromolecules in which n ranges from 10,000 to 100,000 (molecular weight 2*105 to 3 *106 ).
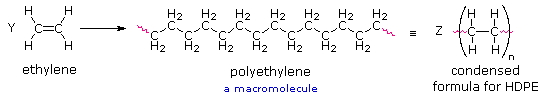
If Y and Z represent moles of monomer and polymer respectively, Z is approximately 10-5 Y. This polymer is called polyethylene rather than polymethylene, (-CH2-)n, because ethylene is a stable compound (methylene is not), and it also serves as the synthetic precursor of the polymer. The two open bonds remaining at the ends of the long chain of carbons (colored magenta) are normally not specified, because the atoms or groups found there depend on the chemical process used for polymerization. The synthetic methods used to prepare this and other polymers will be described later in this chapter.
Unlike simpler pure compounds, most polymers are not composed of identical molecules. The HDPE molecules, for example, are all long carbon chains, but the lengths may vary by thousands of monomer units. Because of this, polymer molecular weights are usually given as averages. Two experimentally determined values are common: Mn , the number average molecular weight, is calculated from the mole fraction distribution of different sized molecules in a sample, and Mw , the weight average molecular weight, is calculated from the weight fraction distribution of different sized molecules. These are defined below. Since larger molecules in a sample weigh more than smaller molecules, the weight average Mw is necessarily skewed to higher values, and is always greater than Mn. As the weight dispersion of molecules in a sample narrows, Mw approaches Mn, and in the unlikely case that all the polymer molecules have identical weights (a pure mono-disperse sample), the ratio Mw / Mn becomes unity.
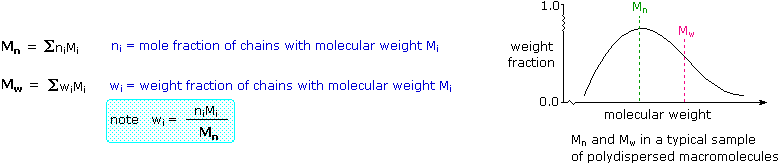
http://www.wwk.in/chemistry/organic-chemistry/polymersApplications of Polymers:Agriculture and Agribusiness
- Polymeric materials are used in and on soil to improve aeration, provide mulch, and promote plant growth and health.Medicine
- Many biomaterials, especially heart valve replacements and blood vessels, are made of polymers like Dacron, Teflon and polyurethane.Consumer Science
- Plastic containers of all shapes and sizes are light weight and economically less expensive than the more traditional containers. Clothing, floor coverings, garbage disposal bags, and packaging are other polymer applications.Industry
- Automobile parts, windshields for fighter planes, pipes, tanks, packing materials, insulation, wood substitutes, adhesives, matrix for composites, and elastomers are all polymer applications used in the industrial market.Sports
- Playground equipment, various balls, golf clubs, swimming pools, and protective helmets are often produced from polymers.
To know more about Polymers click the link given at the end.
ReplyDelete